Regener-Eyes Manufacturer Cited by FDA For Objectionable Conditions and Practices
Inspection Results for Regenerative Processing Plant, LLC Classified as “Official Action Indicated” (Worst Overall Outcome)
Regenerative Processing Plant manufactures Regener-Eyes LITE and Regener-Eyes PRO
In June 2023, the FDA conducted its first inspection of Regenerative Processing Plant LLC’s drug manufacturing facility in Palm Harbor, Florida.
In December 2023, a summary of the results was posted on the FDA’s Data Dashboard. Due to the significant number of objectionable conditions and practices identified by the inspectors, the FDA applied the worst overall outcome of Official Action Indicated.
Dry Eye Foundation obtained a copy of the complete inspection report, called a Form 483, through a Freedom of Information Act request.
Many of the violations cited in this report and on the FDA’s Data Dashboard resemble those listed in the Form 483s of two other OTC ophthalmic manufacturers whose first-time drug facility inspections in 2023 resulted in product recalls: Global Pharma Healthcare Private Limited (manufacturer of EzriCare Artificial Tears, recalled in February) and Kilitch Healthcare India Limited (manufacturer of a large number of generic OTC eye drops, recalled in November.)
Regener-Eyes LITE and Regener-Eyes PRO continue to be recommended and sometimes sold to dry eye patients by their eye care providers. Therefore, as a matter of public safety, we are bringing the results of the FDA inspection to the attention of the Dry Eye Disease patient and provider communities.
Following is our synopsis. Click here to download the synopsis and the FDA Form 483.
-
The findings in Form 483 (attached), both in scope and nature, indicate that Regenerative Processing Plant LLC has failed to:
● properly construct and maintain cleanrooms
● maintain detailed records as required by law
● establish that its manufacturing processes are aseptic (sterile)
● fully investigate abnormal test results that demonstrated bacterial contamination of equipment
● confirm the purity of raw ingredients
-
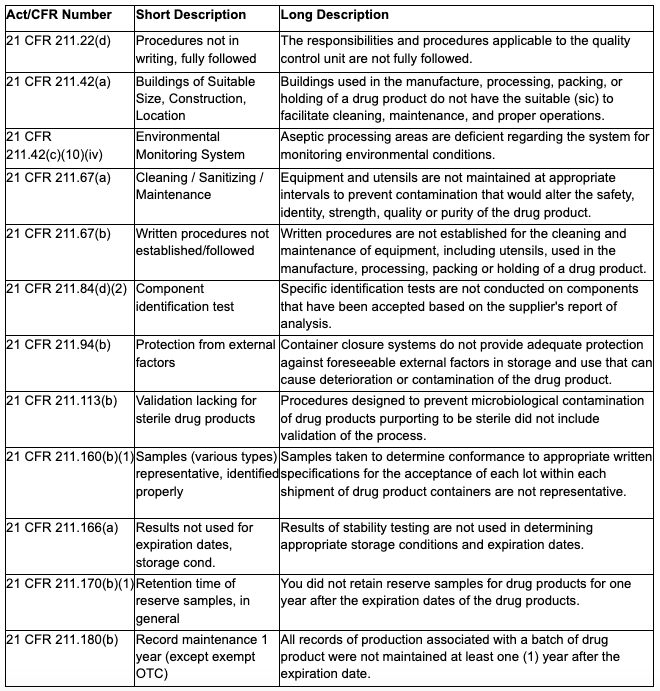
According to the FDA’s Data Dashboard, the Regenerative Processing Plant inspection resulted in citations for violation of 15 Federal regulatory laws. See Appendix 1 for details.
-
Form 483 documents extensive failure to ensure aseptic and accurate manufacturing via process monitoring through the required battery of standard tests. These failures include: tests that were not conducted at all; tests that were improperly conducted; tests that were conducted with unvalidated processes; tests whose results were not documented; tests whose results were ignored; tests whose failed results were not fully investigated; and tests that were prematurely discontinued.
Five “critical deficiencies” were identified in Form 483 regarding quality control. The investigators stated that the list was “not all-inclusive”. (Observation 11)
-
A critical test that ensures that the entire manufacturing process does not introduce contamination is the media fill. In a media fill, a liquid called media that promotes the growth of bacteria and other microorganisms is run through all the equipment used in eye drop manufacturing in the same sequence that the raw ingredients would run through it. If there is any bacterial contamination on the equipment, it will wash off into the media. The media is stored under appropriate conditions to stimulate the growth of microorganisms and is tested periodically. If bacteria or fungal growth is identified, the manufacturer must perform a root cause analysis to determine which piece of equipment was at fault, comprehensively sterilize that device and all downstream equipment, and then repeat the media fill to establish that the machinery has been adequately sanitized and the entire manufacturing process is aseptic (does not introduce microorganisms into the finished product).
Form 483 states that batches of Regener-Eyes PRO and Regener-Eyes LITE that were manufactured both before and after failed or invalidated media fills were released for commercial distribution. (Observations 1, 11)
-
Form 483 states that 27% of all manufactured batches had a pH out of range, with readings from 7.7 to as high as 8.3. Nonetheless, these units were released for commercial distribution. In addition, units from batches whose glycerin content tested out of range were released for commercial distribution. (Observation 2)
-
Observations 5 and 7 document extensive deficiencies in the design, construction and maintenance of the cleanrooms. These include walls and ceiling of unsuitable construction; lack of written procedures for sanitizing surfaces; lack of validation that the cleaning processes in use are actually effective; and lack of proper air monitoring for particulates and pathogens.
In one cleanroom, inspectors observed that slats had been cut into the lower part of one wall to simulate the unidirectional air flow required in a cleanroom. These slats opened into an unmonitored area outside the cleanroom, allowing the influx of particulate-laden air into the cleanroom.
-
Regener-Eyes’ stated storage temperature (room temperature) and shelf life (24 months) are not supported by Regenerative Processing Plant’s own stability testing data. (Observation 3)
-
Regener-Eyes LITE and Regener-Eyes PRO are packaged in a bottle which is “not for single use nor does it have a backflow prevention design to prevent microbial contamination of the product after being shipped.” (Observation 4)
-
In Observation 9, investigators state that Regenerative Processing Plant reported to the FDA that Regener-Eyes LITE and Regener-Eyes PRO containing amniotic fluid were discontinued in June 2021.
See Appendix 2 for three different ingredient lists declared by Regenerative Processing Plant in its official drug listings for Regener-Eyes LITE and Regener-Eyes PRO in the past two years.
-
No bottles of amniotic fluid eye drops were retained as required by law, and no records related to these products could be located. The Chief Strategist stated that she “could not locate any records such as complaint records, distribution records, receiving records, storage records, and manufacturing records” and that she “could not remember where the records went or if they were destroyed or not”. (Observations 9, 11, 14)
Concluding notes from Dry Eye Foundation
Based on FDA’s inspection findings, it is clear that there is no assurance that Regener-Eyes PRO and Regener-Eyes LITE are manufactured in a correctly formulated and sterile fashion. We therefore urge the Dry Eye Disease community to avoid Regener-Eyes eye drops and we urge providers to stop recommending them.
Sincerely,
Sandra Brown MD (Medical Advisor)
Rebecca Petris (Co-Executive Director)
Aidan Moore (Co-Executive Director)
Appendix 2
Ingredient Lists for Regener-Eyes LITE and Regener-Eyes PRO
* This ingredient has no Unique Ingredient Identifier; it appears on product packaging but not on the formal drug label; it is identifiable in medical literature and patent applications as amniotic fluid.
** This ingredient has no Unique Ingredient Identifier; it appears on product packaging but not on the formal drug label; it remains an unknown substance.
*** We consulted the FDA’s Office of Drug Information for information about this ingredient and they stated that it was simply the company’s proprietary name for sodium chloride (salt). We have requested an unredacted version of Form 483 in order to verify this, due in part to the interaction between FDA and Regenerative Processing Plant described in Form 483’s Observation 15.